Review Article
Eugenio Ragazzi*
Eugenio Ragazzi*
Studium Patavinum, University of Padova, 35122 Padova, Italy.
E-mail: eugenio.ragazzi@unipd.
Received: 2025-08-12 | Revised:2025-08-30 | Accepted: 2025-08-30 | Published: 2025-08-31
Pages: 121-136
DOI: https://doi.org/10.56717/jpp.2025.v04i02.043
Abstract
Glaucoma is a progressive
optic neuropathy and a leading cause of irreversible blindness, characterized
by retinal ganglion cell loss and optic nerve degeneration. While elevated
intraocular pressure (IOP) is the main modifiable risk factor, disease
progression can occur independently of IOP, implicating oxidative stress,
inflammation, mitochondrial dysfunction, and extracellular matrix remodeling in
its pathogenesis. MicroRNAs (miRNAs), small non-coding RNAs that regulate gene
expression, have emerged as promising modulators of these pathways and
potential therapeutic targets, although clinical application remains limited by
delivery challenges and variable findings. Emerging evidence suggests that
certain natural compounds and plant-derived bioactive agents can influence
miRNA activity, with potential neuroprotective effects. Although direct
evidence in glaucoma is still limited, studies in related neurodegenerative and
inflammatory conditions indicate a promising therapeutic avenue. This review
discusses the potential of miRNA-targeted phytotherapy as a novel strategy to
modulate key pathogenic pathways and enhance neuroprotection in glaucoma.
Future research should focus on standardized methodologies and robust clinical
validation to translate these findings into therapeutic applications.
Abstract Keywords
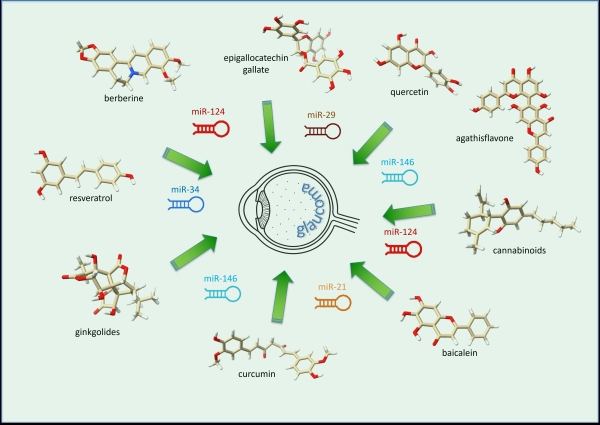
MicroRNA, glaucoma, flavonoids, resveratrol, curcumin, cannabinoids, berberine, Ginkgo biloba.
1.
Introduction
Glaucoma is a progressive optic neuropathy characterized by irreversible retinal ganglion cell (RGC) loss and visual field defects [1]. While elevated intraocular pressure (IOP) remains the primary modifiable risk factor, disease progression can occur even with normal IOP, particularly in normal-tension glaucoma [2]. Beyond mechanical stress, accumulating evidence implicates mitochondrial dysfunction, oxidative stress, inflammation, fibrosis, and extracellular matrix (ECM) remodeling as central to the pathophysiology of glaucomatous neurodegeneration [1,3].
Although IOP regulation remains the cornerstone of glaucoma management, increasing attention is now being directed toward neuroprotective strategies due to the limitations of conventional treatments in halting disease progression [4]. Recent findings from Mendelian randomization and cross-sectional studies have identified high-fat, high-calorie dietary patterns as risk-enhancing, whereas antioxidant-rich diets and optimal sleep appear to be protective [5]. These associations align with emerging mechanistic insights into the role of aging and cellular senescence, especially involving mitochondrial decline, oxidative imbalance, and chronic inflammation, in promoting RGC vulnerability and optic nerve damage [6]. Together, these findings suggest that lifestyle and dietary modulation may beneficially influence the molecular pathways underlying glaucoma pathogenesis.
Among emerging
therapeutic avenues, microRNAs (miRNAs)—small
non-coding RNAs, of 19–22 nucleotides in length (Fig. 1) that fold into short
stem-loops (hairpin) structures, and that regulate gene expression [9] are gaining attention for their involvement in
several pathologies [10], including diabetes
[11,12] and diabetic retinopathy [13]. Their dual potential as both diagnostic
biomarkers and therapeutic targets makes them especially promising, including
key glaucomatous processes [14-18]. In
glaucoma pathogenesis, several miRNAs have emerged as potential therapeutic
targets because of their involvement in key disease mechanisms (supplementary Table
S1). Dysregulation of
specific miRNAs has been linked to trabecular meshwork fibrosis (e.g., miR-29),
TGF-β signaling,
oxidative stress responses, apoptosis, and neurodegeneration. miR-24, miR-29b,
miR-200c, miR-204, miR-143-3p, miR-1260b and miR-125b-5p have been identified
in aqueous humor (AH) and linked to
glaucoma-related molecular mechanisms including gene regulation, IOP
modulation, and retinal degeneration. In the trabecular
meshwork (TM), miRNAs influence ECM remodeling, apoptosis, and cellular
senescence, which are critical for aqueous humor outflow and IOP regulation. In
the retina, miRNAs are implicated in RGC death and axon regeneration,
contributing to glaucomatous vision loss [16].
Dysregulation of several miRNAs, including miR-9, miR-21, miR-23a, miR-27a,
miR-126, miR-144, miR-146, miR-150 and miR-155, has been associated with
retinal diseases, with these alterations most frequently observed in biological
samples from patients with age-related macular degeneration [19]. Despite the therapeutic promise of
miRNA-based strategies, their clinical translation is hindered by challenges in
delivery and a lack of consistent miRNA findings across studies. Notably,
according to Greene et al. [16], only eight
miRNAs (miR-143, miR-221, miR-486, miR-4725, miR-125b, miR-451a, miR-92a, and
miR-99b) were identified in multiple independent studies as having a role in
glaucoma, suggesting that these may represent the most robust and promising
targets for further investigation. Using Ingenuity Pathway Analysis (IPA), the
authors [16] were able to link the
identified miRNAs to autophagy, apoptosis, senescence and neuroinflammation
pathways linked to glaucoma development. Variability in models, sample
demographics, and glaucoma subtypes likely contributes to these discrepancies,
underscoring the need for standardized research to validate these candidates
across diverse populations.
For instance, given its critical role in regulating extracellular matrix genes, the miR-29 family has garnered attention for its anti-fibrotic function in glaucoma [20]. Downregulation of miR-29 isoforms, particularly miR-29a and miR-29c, has been observed in glaucomatous TM and lamina cribrosa cells, and restoring their expression reduces collagen production and TGF-β–mediated fibrosis. However, under oxidative stress, miR-29b-3p may be paradoxically upregulated, contributing to cellular damage and suggesting that context-dependent modulation, rather than uniform upregulation, may be required to achieve therapeutic benefits.
Figure 1. Structure of a typical microRNA.
A) Nucleotide sequence and predicted stem–loop
structure of the Homo sapiens miR-146b precursor (pre-miR-146b). The two
mature miRNAs derived from the opposite arms of the hairpin are indicated:
miR-146b-5p (blue), originating from the 5′ arm, and miR-146b-3p (red), from
the 3′ arm. A, G, C, and U represent the four ribonucleotides adenine, guanine,
cytosine, and uracil, respectively. This scheme was adapted from [7]. Additional structural information is
available at miRBase (https://mirbase.org/hairpin/MI0003129#hsa-miR-146b-5p,
accessed on August 10, 2025).
B) Optimal secondary structure of pre-miR-146b according to minimum free energy (MFE; left) and centroid model (right), predicted using the RNAfold WebServer (http://rna.tbi.univie.ac.at/, accessed on August 10, 2025) [8].
Phytochemicals and herbal extracts with potent antioxidant, anti-inflammatory, and anti-fibrotic properties have been increasingly recognized for their ability to modulate miRNA expression and the downstream gene networks they regulate [12]. These natural compounds can influence key molecular pathways involved in inflammation, oxidative stress, and tissue remodeling, thereby contributing to the prevention or mitigation of various chronic diseases. For example, plant-derived polyphenols have been shown to alter the expression of specific miRNAs in human tissues, modulating inflammatory responses and regulating obesity-related biomarkers [21].
Given the large and growing interest in miRNAs as modulators of gene expression in health and disease, this review explores the potential of natural products, particularly plant-derived compounds, as adjunctive therapies for glaucoma by modulating miRNA activity. Although direct experimental or clinical evidence specifically linking herbal miRNA modulation to glaucoma treatment is still lacking, accumulating studies in related fields, such as neurodegenerative disorders [22], suggest promising avenues for therapeutic intervention. These findings support the hypothesis that targeting disease-relevant miRNAs with phytochemicals may provide a novel and biologically plausible strategy for modulating the molecular mechanisms underlying glaucomatous damage, including RGC loss, oxidative stress, and aberrant tissue remodeling.
2.
Materials and methods
To prepare this review, a comprehensive literature search was performed in the PubMed, Scopus, and Web of Science databases, covering publications available up to August 1, 2025. The search strategy combined terms related to miRNAs and glaucoma with those referring to natural compounds, including phytochemicals and plant-derived molecules. Particular attention was given to studies examining the role of miRNAs in glaucoma pathophysiology and other neurodegenerative diseases, as well as those investigating their modulation by natural agents with potential neuroprotective effects. Both original experimental reports and relevant review articles were considered, and additional references were identified by screening the bibliographies of the key publications. The aim was not to provide a systematic analysis, but rather to highlight and critically discuss the most relevant evidence to date, with a focus on the emerging concept of miRNA modulation by natural compounds as a potential therapeutic strategy for glaucoma. However, the available evidence is still limited and heterogeneous, reflecting the early stage of research in this field.
3.
Results and discussion
3.1. miRNA regulation by
natural compounds: impact on glaucoma
3.1.1. Ginkgo
Ginkgo biloba L. extract (GBE), obtained from the present-day Ginkgo tree, a genus whose lineage dates back over 170 million years, has long been used in traditional Eastern medicine for conditions like asthma, vertigo, and circulatory disorders [23, 24]. Modern research, as highlighted by Lee et al. [4], supports its efficacy in treating cognitive impairments and dementia, owing to its neuroprotective, antioxidant, and anti-inflammatory properties [25]. These benefits are particularly relevant to RGCs, making GBE a potential therapeutic agent for glaucoma [25-27]. Interestingly, while GBE has been shown to improve ocular blood flow and visual field damage [28], its precise impact on visual field progression remains inconclusive [27]. A promising emerging avenue involves exploring the effects of GBE at the molecular level, particularly through the modulation of miRNAs. Given that miRNAs play a critical role in regulating gene expression linked to neuroinflammation, oxidative stress, and apoptosis, GBE's bioactive compounds may exert part of their therapeutic effects by influencing miRNA expression patterns. This miRNA-mediated mechanism may help explain the cellular neuroprotection observed with GBE and provides a novel framework for understanding and optimizing its role in glaucoma treatment.
Neuroinflammation is a key factor in the development of neurodegenerative diseases like Alzheimer’s disease and glaucoma [29]. GBE, traditionally used for blood circulation disorders, has been investigated for its anti-neuroinflammatory effects. Liu et al. [30] demonstrated that GBE significantly inhibited lipopolysaccharide (LPS)-induced inflammation in microglial and glial cells without toxicity. Mechanistically, GBE increases the expression of miR-146b-5p, that post-transcriptionally regulates gene expression. miR-146b-5p exerts its effect by binding to the 3′ untranslated region (3′-UTR) of specific target mRNAs, a region that does not code for proteins but plays a crucial role in the regulation of mRNA stability and translation. One such target is TNF receptor-associated factor 6 (TRAF6), an adaptor protein involved in innate immune and inflammatory signaling via activation of the NF-κB pathway. Upon binding of miR-146b-5p to the 3′-UTR of TRAF6 mRNA, translation of TRAF6 protein is suppressed, leading to decreased intracellular levels of TRAF6 [30]. This, in turn, results in the attenuation of NF-κB activation (Fig. 2), a key transcription factor complex that drives the expression of various pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α, and COX-2. Supporting this mechanism, Zhou et al. [31] demonstrated in experimental glaucoma that the closely related miR-146a-5p directly targets both TRAF6 and interleukin-1 receptor-associated kinase 1 (IRAK1), leading to the downregulation of the IRAK1/TRAF6/NF-κB signaling axis in microglia, which reduces neuroinflammation and protects against retinal ganglion cell injury. Collectively, these findings indicate that GBE exerts anti-inflammatory effects via the miR-146b-5p/TRAF6/NF-κB pathway and highlight the broader therapeutic potential of miR-146 family members in neuroinflammatory conditions.
Figure 2. Proposed mechanisms by which miR-146a-5p and miR-146b-5p modulate inflammation in glaucoma.
Red bars indicate inhibitory actions. Key mediators involved in inflammatory signaling pathways relevant to glaucoma are shown: TLR: Toll-like receptor; MyD88: Myeloid differentiation primary response protein 88; IRAK1: Interleukin-1 receptor-associated kinase; TRAF6: TNF receptor-associated factor 6; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells. On the right, the sequences of miR-146a-5p and miR-146b-5p are shown.
Additional insights are provided by findings on miR-146b [7], the primary transcript from which the functional strand miR-146b-5p is derived. In the tears of glaucoma patients [16, 32], the expression of miR-146b appears to be increased, compared to that in healthy subjects; the increased level of miR-146b in the tears of glaucoma patients could represent a compensatory defensive mechanism. miR-146b is well-known for its role as a negative regulator of inflammation, as above mentioned, primarily by targeting key signaling molecules like TRAF6 and downregulating the NF-κB pathway, which controls the expression of many pro-inflammatory cytokines [30, 33]. In the context of glaucoma, where chronic neuroinflammation contributes to retinal ganglion cell damage and disease progression, the upregulation of miR-146b might be the body's attempt to counteract excessive inflammation and protect neural tissues. Thus, elevated miR-146b levels in the tear fluid may reflect an endogenous response intended to limit inflammatory damage and restore immune balance in ocular tissues affected by glaucoma. However, if this compensatory mechanism is insufficient or overwhelmed, neurodegeneration can still progress. This also opens avenues for therapies that boost miR-146b activity, such as GBE, to support the natural defense system.
3.1.2. Flavonoids
Flavonoids, a diverse group of polyphenolic compounds found in many fruits, vegetables, and medicinal plants, besides their role as anti-inflammatory agents and combating neurodegenerative diseases [34], have emerged as potential modulators of miRNA expression, including the anti-fibrotic miR-29 family, in various pathological conditions [22, 35, 36]. In the context of glaucoma—particularly primary open-angle glaucoma (POAG), where fibrosis at the lamina cribrosa and trabecular meshwork contributes to disease progression—modulation of miR-29 is of significant interest [20], since a dysregulation of miR-29b is believed to contribute to abnormal deposition of extracellular matrix [18]. The miR-29 family is known to suppress the expression of ECM components such as collagens and fibronectin, which are upregulated by transforming growth factor-beta (TGF-β), a key driver of glaucomatous damage [37]. Therefore, miR-29 family is considered as anti-fibrotic through its effects on the TGF-β signaling pathway, modulating the production and deposition of extracellular matrix [20, 36] (Fig. 3). However, Liu et al. [38] suggested that inhibiting miR-29b-3p may protect against glaucoma by reducing apoptosis, oxidative stress, and extracellular matrix accumulation, while enhancing human trabecular meshwork (hTM) cell survival under oxidative stress. These effects appear to involve the upregulation of E3 ubiquitin-ligase RNF138 and subsequent activation of the extracellular signal-regulated kinase (ERK) signaling pathway, indicating a potential new therapeutic approach for glaucoma (Fig. 3).
Figure 3. Dual role of miR-29 Family in glaucoma: Anti-fibrotic (left) vs context-dependent pro-survival pathways (right).
ECM: extracellular matrix; RNF138: E3 ubiquitin‑ligase RNF138; TGF-β: transforming growth factor-beta; ERK: extracellular regulated protein kinase; TM: trabecular meshwork.
Flavonoids, such as epigallocatechin gallate (EGCG, a catechin) and quercetin (a flavonol) from green tea, and genistein (an isoflavone) from soy, have been shown in various experimental models to upregulate miR-29 expression [39-42], suggesting the inhibition of TGF-β signaling and reduction of ECM deposition. Zhou et al. [43], using a mouse myoblast model, showed that miR-29 suppresses fibrogenic differentiation by downregulating extracellular matrix and cell adhesion genes, suggesting that its upregulation, potentially achievable through flavonoids like quercetin, could help counteract fibrotic changes in glaucomatous tissues. This indicates the potential for therapeutic applications in diseases like glaucoma, where fibrosis plays a key role. Although direct experimental studies on glaucomatous tissues are still lacking, these findings suggest that flavonoid-induced upregulation of miR-29 could represent a novel therapeutic avenue to counteract fibrosis in glaucoma. Further research is warranted to explore the specific effects of dietary or pharmacological flavonoids on miR-29 expression in ocular tissues and their potential to modify disease outcomes in glaucoma patients.
Quercetin has been demonstrated to have a complex influence on multiple miRNA [44]. In particular, its ability to induce miRNA-146a has been linked to anti-inflammatory actions [45]. Agathisflavone (biflavonoid), another bioflavonoid purified from Cenostigma pyramidale (Tul.) Gagnon and Lewis, exhibited strong anti-inflammatory and neuroprotective effects by modulating microglial activation and reducing pro-inflammatory mediators such as IL-1β, IL-6, and NOS2 [46, 47]. In particular, agathisflavone downregulates miR-146a and miR-155 expression in microglia activated by LPS or β-amyloid, suggesting a regulatory effect on miRNA-driven inflammatory pathways. While miR-146a is generally considered a feedback inhibitor of inflammation, its sustained upregulation in chronic neuroinflammation may contribute to dysregulated immune responses. In glaucoma, a neurodegenerative disease with significant inflammatory component, miR-146a has been implicated in modulating microglial reactivity and RGC survival. The ability of agathisflavone to normalize miR-146a expression under inflammatory conditions suggests a potential role in controlling microglial dysfunction in glaucoma, supporting its promise as a novel therapeutic candidate for inflammatory neurodegenerative diseases affecting the eyes.
Another interesting phytoconstituent is baicalein, a flavone originally isolated from Scutellaria baicalensis Georgi roots, which exhibits multiple neuroprotective properties, including activity on γ-aminobutyric acid (GABA) type Receptors, a mechanism previously linked to cognitive improvement and neuroprotection in models of Alzheimer’s disease [48]. Given the growing evidence that GABAA modulation contributes to neuronal survival, synaptic stability, and reduced excitotoxicity, baicalein’s GABAergic effects may also be relevant in the context of glaucomatous retinal neurodegeneration. Moreover, in vitro studies by Li et al. [49] demonstrated that baicalein relaxes cultured hTM cells by reducing myosin light chain phosphorylation and modulating ECM regulatory proteins. These cellular changes translated into a significant increase in outflow facility (by approximately 90%) in enucleated mouse eyes perfused ex vivo, corresponding to an estimated ~5 mmHg reduction in IOP when extrapolated to in vivo conditions. Although these findings are based on in vitro and ex vivo models, they support the mechanistic potential of baicalein in lowering IOP through trabecular meshwork modulation. Beyond its biomechanical effects, baicalein has been shown to modulate key miRNAs implicated in glaucoma pathophysiology. Notably, baicalein suppresses miR-21, a miRNA known to promote TGF-β1/Smad signaling and fibrotic remodeling in various tissues [50]. In fibrotic disease models, baicalein-mediated inhibition of miR-21 attenuates TGF-β1 activity, reduces collagen deposition, and reverses myofibroblast differentiation [51], suggesting that similar anti-fibrotic actions could be beneficial in glaucomatous eyes (Fig. 4). However, miR-21 also appears to play a protective role in glaucoma in certain contexts. For example, Su et al. [52] demonstrated that miR-21a-5p, a mature form of the miR-21 family, contributes to mesenchymal stem cell-mediated neuroprotection in acute glaucoma by modulating the PDCD4/stanniocalcin-1 (STC1) axis. Similarly, Tan et al. [53] reported that miR-21-5p delivered via PDA/PEI nanoparticles reduced intraocular pressure by enhancing aqueous outflow through a mechanism involving endothelial nitric oxide synthase. These findings highlight the context-dependent functions of miR-21 in glaucoma and suggest that its modulation by baicalein should be carefully evaluated, balancing its potential anti-fibrotic benefits with its possible neuroprotective and IOP-lowering effects. Moreover, baicalein upregulates miR‑124 in RGCs, leading to the suppression of inflammatory chemokines such as MCP‑1 and offering neuroprotective benefits under stress conditions [54]. Together, these findings suggest that baicalein may exert dual therapeutic effects in glaucoma, by lowering IOP through TM cell relaxation and ECM remodeling, and by protecting RGCs via anti-inflammatory miRNA pathways. In line with this, Xiao et al. [55] and Zhao et al. [56] emphasized that the flavones of Scutellaria radix, including baicalein, exhibit multiple routes of therapeutic action, with diverse pharmacological properties and mechanisms demonstrated in cellular and animal models of ocular diseases.
Figure 4. Theoretical mechanism of action for baicalein, berberine and curcumin in glaucoma via miR-21 modulation.
Red bars indicate the inhibitory actions. IOP: intraocular pressure; PDCD4: programmed cell death protein 4; STC1: stanniocalcin- 1; TGF-b1: transforming growth factor beta 1.
As a general consideration, it is important to acknowledge the dual role of flavonoids: depending on their specific structure, concentration, and cellular context, they can exert both antioxidant and pro-oxidant effects [57]. Flavonoids are widely recognized for their antioxidant properties, such as neutralizing free radicals and reducing oxidative stress, however, some may act as pro-oxidants under certain conditions, potentially contributing to oxidative damage. Dysregulation of specific miRNAs is linked to oxidative stress, inflammation, and angiogenesis, all of which are involved in ocular diseases [19]. The dual antioxidant and pro-oxidant properties of flavonoids should be considered when evaluating their influence on miRNA expression, as their effects may vary and potentially impact the neuroprotective mechanisms relevant to glaucoma.
3.1.3. Resveratrol
Resveratrol (3,4',5-trihydroxystilbene), a natural polyphenol contained in several plants, like grapes, has demonstrated significant neuroprotective effects in experimental models of glaucoma, as confirmed by a 2025 systematic review and meta-analysis by Zhang et al. [58], which analyzed 30 preclinical studies. The authors found that resveratrol significantly increased sirtuin 1 (SIRT1) expression, enhanced RGC survival, and slowed retinal thinning, with improvements in overall visual function. These effects appear to be mediated by the suppression of inflammatory cytokines and cell apoptosis. Despite these promising results, the authors cautioned that differences in disease complexity and administration routes between animal models and clinical practice warrant further translational research to establish effective dosing and delivery strategies for human use.
Resveratrol has been reported to exert divergent regulatory effects on miR-34a depending on the pathological context. In cardiomyocyte injury models, resveratrol downregulates miR-34a, thereby relieving its inhibitory effect on SIRT1, which promotes mitochondrial function, reduces oxidative stress, and attenuates inflammation [59, 60]. These mechanisms—miR-34a suppression leading to SIRT1 restoration—are highly relevant to glaucoma, where oxidative stress, mitochondrial dysfunction and RGC death are central features. Conversely, in cancer cells, notably in ovarian and colorectal models, resveratrol has been shown to upregulate miR-34a, enhancing apoptosis by targeting BCL 2, a known anti-apoptotic factor [61]. This suggests a tissue- and pathology-specific modulation of miR-34a by resveratrol, suppressing miR-34a in degenerative/inflammatory settings while upregulating it in oncogenic contexts to promote apoptosis in cancer cells. Given that SIRT1 activation and mitochondrial protection are beneficial in glaucoma (Fig. 5), it is reasonable to hypothesize that resveratrol may exert similar protective effects in ocular tissues via downregulation of miR-34a, although direct evidence from glaucoma models is currently lacking.
Figure 5. Proposed mechanism of resveratrol action in glaucoma via miR-34a/SIRT1 modulation.
Resveratrol is hypothesized to exert neuroprotective effects in glaucoma by downregulating miR-34a, thereby relieving its inhibitory effect on SIRT1 (Sirtuin 1). SIRT1 activation promotes mitochondrial function, reduces inflammation and oxidative stress, and may ultimately protect retinal ganglion cells (RGC) from degeneration. While this mechanism has been demonstrated in non-ocular degenerative models, its relevance to glaucoma remains a promising area for further investigation. Red bars indicate inhibitory action.
Dose dependency is a key consideration for
translating resveratrol’s neuroprotective and miR-34a modulatory effects into
glaucoma therapy. In preclinical glaucoma models, an optimal protective
efficacy was observed with moderate cumulative doses of 160–240 mg/kg, whereas doses below 80 mg/kg were suboptimal, and
higher doses did not confer additional benefit [58]. Specific studies using single day intraperitoneal
doses of 20–40 mg/kg/day in rodents over short periods (5–8 days) effectively increased RGC survival, elevated SIRT1 levels, reduced
pro-apoptotic markers, and maintained retinal thickness [58]. While human trials have administered resveratrol
up to 5 g/day with tolerable safety, doses above 500 mg/day have been associated
with gastrointestinal side effects, underscoring the need for cautious dose
selection in ocular translational studies [62]. Therefore, for future glaucoma-focused
investigations of resveratrol’s impact on miR-34a/SIRT1 signaling, a dose
response design is crucial. Starting with the translational equivalent of 30 mg/kg/day (~150 mg/day in humans) and
exploring within the effective animal range (160–240 mg/kg total) may help identify the minimal effective dose that balances
efficacy, target engagement, and safety of this compound.
While preclinical studies consistently show that resveratrol enhances SIRT1 expression in retinal and neural tissues, the translation of this effect to humans remains uncertain. A meta-analysis by Mansouri et al. [63] found no significant increase in SIRT1 protein levels following resveratrol supplementation in human subjects. However, subgroup analyses hinted at a possible dose- and duration-dependent effect, suggesting that insufficient dosing or short-term interventions may underlie the lack of measurable SIRT1 response. These findings emphasize the need for rigorous clinical trials to explore optimal dosing regimens, treatment durations, and population-specific responses to clarify whether resveratrol’s molecular effects, such as SIRT1 activation and miR-34a modulation, can be harnessed therapeutically in glaucoma.
3.1.4. Curcumin
Curcumin (diferuloylmethane), a natural polyphenol extracted from the rhizome of Curcuma longa L., has emerged as a potential neuroprotective agent in various models of neurodegenerative diseases [64], including glaucoma and other eye diseases [65], partly through its modulation of inflammatory pathways, in particular NF-κB and Toll-like receptor 4 (TLR4) [66]. Curcumin has also been suggested for clinical applications in ophtalmology [66-68]. Pretreatment with curcumin enhanced the survival of BV-2 murine microglia cells, reduced oxidative stress markers, and limited apoptotic activity. Moreover, in a rat model mimicking chronic ocular hypertension, curcumin prevented RGC death and inhibited pro-apoptotic factors, indicating a protective role against high intraocular pressure-induced damage [69]. In another glaucoma-relevant model (retinal ischemia followed by reperfusion in rats) curcumin supplementation helped preserve retinal integrity by modulating mitochondrial dynamics and oxidative stress responses [70]. These findings suggest that curcumin may protect against glaucomatous neurodegeneration by regulating mitochondrial homeostasis and oxidative damage, pathways that may overlap with miRNA-dependent mechanisms. One key mechanism could involve the upregulation of miR-146a [31,71], which discussed above, is a known negative regulator of the NF-κB signaling pathway. In glioblastoma cells, Wu et al. [72] demonstrated that curcumin significantly increased miR-146a expression, which subsequently suppressed NF-κB activity and enhanced apoptotic sensitivity to the alkylating agent temozolomide. Although this study was conducted in a cancer model, the molecular implications are relevant for glaucomatous neurodegeneration, where chronic inflammation and NF-κB activation contribute to retinal ganglion cell loss. Thus, curcumin’s ability to modulate miR-146a and downstream NF-κB signaling may represent a promising therapeutic strategy to counteract neuroinflammation and protect retinal neurons in glaucoma.
In addition to its effects on miR-146a and NF-κB, curcumin has also been shown to modulate miR-21 (considered a key regulator of TGF-β1 signaling and inflammation [50, 73]), as discussed above in relation to baicalein. Tang et al. [74] demonstrated that low-dose curcumin significantly downregulated miR-21 expression and IL-6 levels while restoring regulatory T cell populations in peripheral blood mononuclear cells from patients with myocardial infarction, highlighting curcumin’s anti-inflammatory and immunomodulatory potential. The relevance of miR-21 to glaucoma is supported by Tan et al. [53, 75], who showed that topical application of miR-21-5p, belonging to the miR-21 family, mimics significantly reduced IOP and improved outflow facility in mice by targeting genes involved in cytoskeletal dynamics, extracellular matrix remodeling, and endothelial permeability, including SMAD7, FGF18, and TIMP3/MMP9. These findings suggest a dual role for miR-21 in ocular physiology: while its upregulation may enhance aqueous humor outflow and lower IOP, sustained overexpression is also linked to TGF-β1-driven fibrotic changes in trabecular meshwork cells (Fig. 4). Therefore, curcumin’s ability to fine-tune miR-21 expression may allow it to restore physiological balance by reducing pro-fibrotic signaling while preserving or enhancing outflow facility. This complex regulatory capacity positions curcumin, potentially in association with baicalein, as an adjunct therapy for glaucoma, capable of acting on multiple molecular targets also via miRNA modulation.
3.1.5. Cannabinoids
Cannabis-derived compounds such as the non-psychoactive cannabidiol (CBD) and the psychoactive Δ⁹‑tetrahydrocannabinol (THC) have attracted attention for their potential neuroprotective and anti‑inflammatory effects [76] beyond their modest intraocular pressure-lowering actions [77]. One key route of interest is the modulation of miR‑146a (specifically in the mature form miR-146a-5p), that functions as a negative-feedback regulator of NF‑κB–mediated inflammation [71] (Fig. 2). In murine BV‑2 microglial cell cultures, CBD at 10 μM strongly downregulated miR‑146a expression under resting conditions and inhibited LPS-induced upregulation of miR‑146a, accompanied by reduced IL‑6 and IL‑1β release and suppression of NF‑κB activation [78]. As miR‑146a ordinarily acts to curb excessive inflammatory signaling via targeting IRAK1 and TRAF6, CBD’s modulation appears to fine-tune but ultimately limit neuroinflammatory cascades. While THC showed less robust effects when tested alone, combined treatment with THC and CBD in experimental autoimmune encephalomyelitis (EAE) mice produced a broader modulation of inflammatory miRNAs, significantly downregulating miR-21a-5p, miR-31-5p, miR-122-5p, miR-146a-5p, miR-150-5p, miR-155-5p, and miR-27b-5p, while upregulating miR-706-5p and miR-7116 [79]. These changes were associated with the suppression of Th17/Th1 responses, reduction of pro-inflammatory cytokines (IL-17, IFN-γ, TNF-α, IL-1β and IL-6), and an increase in anti-inflammatory mediators such as IL-10, IL-4, and TGF-β. Although direct evidence in glaucoma models is lacking, these findings suggest that CBD, and particularly THC/CBD combinations, could mitigate chronic retinal neuroinflammation by modulating miRNA-regulated immune pathways, with the potential to protect retinal ganglion cells from inflammatory injury. Nonetheless, as highlighted by recent reviews [80], despite early reports of IOP-lowering effects, the therapeutic role of cannabinoids in glaucoma remains uncertain, limited by a short duration of action, inadequate ocular formulations, and the absence of well-controlled clinical studies. Thus, the potential value of cannabinoids in glaucoma may depend less on their modest IOP-lowering properties and possibly more on their ability to influence miRNA networks involved in neuroinflammation, immune signaling, and retinal ganglion cell survival.
3.1.6. Berberine
Berberine, an isoquinoline quaternary alkaloid found in plants of Berberis and Coptis genera, exhibits pronounced neuroprotective, anti-inflammatory, and antioxidant properties [81], making it a compound of interest in glaucoma [82], where oxidative stress and RGC degeneration play central roles. Although direct studies of berberine on glaucoma via miRNA modulation are lacking, available research supports its plausible effects on miR‑124, a miRNA with strong anti-inflammatory and neuroprotective activity in retinal tissue [83, 84]. In a model of retinal injury (transgenic rat overexpressing the human polycystin-2 gene in retinal photoreceptors), delivery of miR‑124 mimics suppressed microglial activation, reduced pro‑inflammatory cytokine expression (in particular TNF‑α, IL‑1β, CCL2 and others), and preserved neuroretinal function [84]. Since berberine has been shown to deactivate NF‑κB signaling and reduce apoptosis in retinal Müller glia under oxidative stress conditions [85], its anti-inflammatory effects may intersect with miR‑124 regulation. While the direct modulation of miR-124 by berberine in ocular models has not yet been demonstrated, berberine influences miRNA networks in other neuroinflammatory contexts and miR-124 is implicated in apoptosis and tissue remodeling. Together with potential interactions with miR-214 [86], berberine may support a dual mechanism of neuroprotection and attenuation of inflammatory and fibrotic processes in glaucoma.
In parallel, berberine has been shown to downregulate miR-21 in cancer models via IL6/STAT3 signaling, leading to increased PDCD4 expression and activation of p53-mediated apoptosis [87, 88]. Translating these findings to glaucoma, miR-21 is involved in both fibrotic remodeling through TGF-β1/Smad pathways and neuroprotective responses by modulating PDCD4/STC1 in RGCs [50–53]. Therefore, berberine’s ability to reduce miR-21 levels could mitigate pathological fibrotic remodeling (Fig. 4) while potentially influencing RGC apoptosis in a context-dependent manner. It should be emphasized that direct in vivo ocular evidence for berberine is currently lacking, and most mechanistic insights have been extrapolated from studies on other neurodegenerative or systemic disease models. Nevertheless, by simultaneously enhancing miR-124–mediated anti-inflammatory neuroprotection, modulating miR-214, and regulating miR-21–dependent fibrotic and apoptotic pathways, berberine may exert complementary mechanisms to preserve RGC function and counter glaucomatous degeneration. Combined with its capacity to cross the blood–brain barrier and counter oxidative stress, inflammation, and apoptosis in preclinical neurodegenerative models [82], berberine emerges as a promising candidate for miRNA-targeted therapeutic strategies in glaucoma, although further studies are required to clarify its ocular bioavailability, effective dosage, and clinical efficacy.
3.1.7. XenomiRs as glaucoma modulators
In addition to modulating host miRNA expression, it has been proposed that miRNAs present in plant‑based foods, termed xenomiRs, may survive processing, persist through digestion, and be detected in human biofluids [89-92]. However, although some studies have reported their presence in faces or even plasma, the overall evidence is highly inconsistent [89]. Many follow-up investigations have failed to replicate these observations, and large-scale re-analyses suggest that most xenomiRs identified in human samples are likely to represent sequencing artifacts rather than genuine dietary uptake [93]. Consequently, the notion of meaningful cross-kingdom miRNA transfer remains speculative and requires rigorous validation. Interestingly, certain reports indicate that edible plants contain miRNAs, such as miR156e, miR159, miR162 and miR168, that can resist degradation and reach the distal gastrointestinal tract [92, 94]. However, strategies to improve their absorption are still necessary for these plant-derived miRNAs to potentially reach host tissues and exert physiological effects. It has also been reported that miRNAs can be absorbed when encapsulated in their natural form as plant-derived exosome-like nanoparticles (PELNs) [95]. The systematic sequence analysis conducted by Zhao et al. [96] of 166 known xenomiRs and 942 non-xenomiRs from commonly consumed plants, revealed that xenomiRs exhibit potential sequence specificity and may be selectively absorbed by the human body. By employing both random forest and convolutional neural network models, the authors predicted 241 additional candidate xenomiRs and analyzed their potential biological functions in humans. Overall, these findings suggest that plant-derived xenomiRs may modulate human molecular pathways, including those implicated in glaucoma. This raises intriguing possibilities for dietary modulation of miRNA-regulated processes in the eye and paves the way for novel therapeutic or preventive strategies targeting glaucomatous neurodegeneration through nutrition-based approaches.
4. Conclusions
Growing evidence supports the involvement of miRNAs in the pathogenesis of glaucoma, highlighting their potential as diagnostic, prognostic, and therapeutic targets. However, much of the current research remains at the experimental stage, often based on in vitro models, and lacks validation through large-scale clinical studies. Whether changes in miRNA expression are causative or consequential in glaucoma progression remains unclear. Nonetheless, the modulation of specific miRNAs by bioactive compounds from medicinal plants, such as flavonoids and alkaloids, offers a promising avenue for future therapeutic strategies. These natural compounds may help restore miRNA homeostasis and counteract the key pathological mechanisms of glaucoma, including oxidative stress, inflammation, and fibrosis. Although compounds such as baicalein, resveratrol, and Ginkgo biloba extract have demonstrated neuroprotective and anti-inflammatory effects in vivo and in some clinical settings, it remains unclear whether these benefits are mediated via miRNA modulation. For other agents, including berberine and most cannabinoids, mechanistic evidence is largely extrapolated from non-ocular models. In addition, aerobic physical exercise has been shown to beneficially modulate CNS-specific miRNAs [97], such as miRNA-124, enhancing neurotrophic signaling and cognitive resilience, suggesting its potential as an adjunctive strategy to support optic nerve health in glaucoma. Moving forward, integrating miRNA profiling with phytotherapy could open new avenues for more targeted and personalized approaches to glaucoma management, although rigorous clinical validation is still required.
Supplementary material
Supplementary material related to this article can be found online at
https://leafletpub.com/images/articlesFile/supplementary.1757227514.pdf
Authors’ contributions
The author confirms sole responsibility for all aspects of the article, including the conception, literature review, analysis, and manuscript preparation.
Acknowledgements
The author has no acknowledgements to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
All data will be made available on request according to the journal policy.
Conflicts of interest
The author declares no conflicts of interest.
References
|
1. |
Ekici, E.; Moghimi, S. Advances in understanding glaucoma
pathogenesis: A multifaceted molecular approach for clinician
scientists. Mol. Aspects Med. 2023, 94, 101223. https://doi.org/10.1016/j.mam.2023.101223 |
|
2. |
Asrani, S.G.; McGlumphy, E.J.; Al-Aswad, L.A.; Chaya, C.J.; Lin,
S.; Musch, D.C.; Pitha, I.; Robin, A.L.; Wirostko, B.; Johnson, T.V. The
relationship between intraocular pressure and glaucoma: An evolving
concept. Prog. Retin. Eye Res. 2024, 103, 101303. |
|
|
https://doi.org/10.1016/j.preteyeres.2024.101303 |
|
3. |
Lin, B.; Li, D. The pivotal role of inflammatory factors in
glaucoma: a systematic review. Front. Immunol. 2025, 16, 1577200.
https://doi.org/ 10.3389/fimmu.2025.1577200 |
|
4. |
Lee, H.P.; Tsung, T.H.; Tsai, Y.C.; Chen, Y.H.; Lu, D.W.
Glaucoma: Current and new therapeutic approaches. Biomedicines. 2024, 12,
2000. https://doi.org/ 10.3390/biomedicines12092000 |
|
5. |
Shengnan, Z.; Tao, W.; Yanan, Z.; Chao, S. Exploring the impact
of diet, sleep, and metabolomic pathways on Glaucoma subtypes: insights from
Mendelian randomization and cross-sectional analyses. Nutr. Metab. 2025, 22,
74. https://doi.org/10.1186/s12986-025-00967-4 |
|
6. |
Zhang, Y.; Huang, S.; Xie, B.; Zhong, Y. Aging, Cellular
Senescence, and Glaucoma. Aging Dis. 2024, 15(2), 546-564. https://doi.org/10.14336/AD.2023.0630-1 |
|
7. |
Garcia, A.; Mazoyer, S. MIR146B (microRNA 146b). Atlas Genet Cytogenet Oncol Haematol. 2012,
2012-12-01. Online version: http://atlasgeneticsoncology.org/
gene/50855/mir146b-%28microrna-146b%29 (accessed 30/07/2025). |
|
8. |
Gruber, A.R.; Lorenz, R., Bernhart, S.H.; Neuböck, R.; Hofacker,
I.L. The Vienna RNA websuite. Nucleic Acids Res. 2008, 36(Web Server issue),
W70–W74. https://doi.org/10.1093/nar/gkn188 |
|
9. |
Bartel, D.P. MicroRNAs: genomics, biogenesis, mechanism, and function.
Cell. 2004, 116(2), 281-297. https://doi.org/10.1016/s0092-8674(04)00045-5 |
|
10. |
Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Therapeutic
advances of miRNAs: A preclinical and clinical update. J. Adv. Res. 2021,
28, 127–138. https://doi.org/10.1016/j.jare.2020.08.012 |
|
11. |
He, X.; Kuang, G.; Wu, Y.; Ou, C. Emerging roles of exosomal
miRNAs in diabetes mellitus. Clin. Transl. Med. 2021, 11(6), e468. https://doi.org/10.1002/ctm2.468 |
|
12. |
Sivri, D.; Gezmen-Karadağ, M. Effects of phytochemicals on type 2
diabetes via microRNAs. Curr. Nutr. Rep. 2024, 13(3), 444-454.
https://doi.org/10.3390/ 10.1007/s13668-024-00549-5 |
|
13. |
Wu, Q.; Liu, C.; Shu, X.; Duan, L. Mechanistic and
therapeutic perspectives of non-coding RNA-modulated apoptotic signaling in
diabetic retinopathy. Cell. Biol. Toxicol. 2024, 40(1), 53.
https://doi.org/10.1007/s10565-024-09896-z |
|
14. |
Molasy, M.; Walczak, A.; Szaflik, J.; Szaflik, J.P.;
Majsterek, I. MicroRNAs in glaucoma and neurodegenerative
diseases. J. Hum. Genet. 2017, 62, 105–112. https://doi.org/10.1038/jhg.2016.91 |
|
15. |
Gasińska, K.; Kosior-Jarecka, E.; Żarnowski, T. MicroRNA in the
pathogenesis of glaucoma. Ophthatherapy. 2020, 7(4), 277-286. https://doi.org/
10.24292/01.OT.311220.1 |
|
16. |
Greene, K.M.; Stamer, W.D.; Liu, Y. The role of microRNAs in
glaucoma. Exp. Eye Res. 2022, 215, 108909. https://doi.org/10.1016/j.exer.2021.108909 |
|
17. |
Zhang, R.; Tao, Y.; Huang, J. The application of microRNAs in
glaucoma research: A bibliometric and visualized analysis. Int. J. Mol.
Sci. 2023, 24(20), 15377. https://doi.org/10.3390/ijms242015377 |
|
18. |
Dobrzycka, M.; Sulewska, A.; Biecek, P.; Charkiewicz, R.;
Karabowicz, P.; Charkiewicz, A.; Golaszewska, K.; Milewska, P.;
Michalska-Falkowska, A.; Nowak, K.; Niklinski, J.; Konopińska, J. miRNA studies
in glaucoma: A comprehensive review of current knowledge and future
perspectives. Int. J. Mol. Sci. 2023, 24(19), 14699. https://doi.org/10.3390/ijms241914699 |
|
19. |
Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Smigielska, B.;
Szaflik, J.P.; Szaflik, J.; Majsterek, I. The role of oxidative stress and
the importance of miRNAs as potential biomarkers in the development of
age-related macular degeneration. processes. 2021, 9, 1328. https://
doi.org/10.3390/pr9081328 |
|
20. |
Smyth, A.; Callaghan, B.; Willoughby, C. E.; O'Brien, C. The role
of miR-29 family in TGF-β driven fibrosis in glaucomatous optic neuropathy. Int.
J. Mol. Sci. 2022, 23(18), 10216. https://doi.org/10.3390/ijms231810216 |
|
21. |
Corrêa, T.A.; Rogero, M.M. Polyphenols regulating microRNAs and
inflammation biomarkers in obesity. Nutrition. 2019, 59, 150–157.
https://doi.org/ 10.1016/j.nut.2018.08.010 |
|
22. |
Ghosh, S.; Kumar, V.; Mukherjee, H.; Lahiri, D.; Roy, P.
Nutraceutical regulation of miRNAs involved in neurodegenerative diseases and
brain cancers. Heliyon. 2021, 7(6), e07262. https://doi.org/10.1016/j.heliyon.2021.e07262 |
|
23. |
Noor-E-Tabassum; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra,
S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.; Hossain, M.J.; Dhama, K.;
Mostafa-Hedeab, G.; Emran, T.B. Ginkgo biloba: A treasure of
functional phytochemicals with multimedicinal applications. Evid. Based
Complement. Altern. Med. 2022, 2022, 8288818. https://doi.org/10.1155/2022/8288818 |
|
24. |
Liu, Y.; Xin, H.; Zhang, Y.; Che, F.; Shen, N.; Cui, Y. Leaves,
seeds and exocarp of Ginkgo biloba L. (Ginkgoaceae): A comprehensive
review of traditional uses, phytochemistry, pharmacology, resource
utilization and toxicity. J. Ethnopharmacol. 2022, 298, 115645.
https://doi.org/10.1016/j.jep.2022.115645 |
|
25. |
Labkovich, M.; Jacobs, E.B.; Bhargava, S.; Pasquale, L.R.;
Ritch, R. Ginkgo biloba extract in ophthalmic and systemic disease,
with a focus on normal-tension glaucoma. Asia Pac. J. Ophthalmol. 2020, 9(3),
215–225. https://doi.org/
10.1097/APO.0000000000000279 |
|
26. |
Pinazo-Duran, M.D.; Shoaie-Nia, K.; Zanon-Moreno, V.;
Sanz-Gonzalez, S.M.; Del Castillo, J.B.; Garcia-Medina, J.J. Strategies to reduce
oxidative stress in glaucoma patients. Curr. Neuropharmacol. 2018,
16(7), 903–918. https://doi.org/10.2174/ 1570159X15666170705101910 |
|
27. |
Kang, J.M.; Lin, S. Ginkgo biloba and its potential role
in glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 116–120.
https://doi.org/10.1097/ICU.0000000000000459 |
|
28. |
Quaranta, L.; Bettelli, S.; Uva, M.G.; Semeraro, F.; Turano, R.;
Gandolfo, E. Effect of Ginkgo biloba extract on preexisting visual
field damage in normal tension glaucoma. Ophthalmology. 2003, 110,
359–362. https://doi.org/10.1016/S0161-6420(02)01745-1 |
|
29. |
Quaranta, L.; Bruttini, C.; Micheletti, E.; Konstas, A.G.P.;
Michelessi, M.; Oddone, F.; Katsanos, A.; Sbardella, D.; De Angelis, G.;
Riva, I. Glaucoma and neuroinflammation: An overview. Surv. Ophthalmol. 2021,
66(5), 693-713. https://doi.org/10.1016/j.survophthal.2021.02.003 |
|
30. |
Liu, M.; Peng, Y.; Che, Y.; Zhou, M.; Bai, Y.; Tang, W.; Huang, S.;
Zhang, B.; Deng, S.; Wang, C.; Yu, Z. MiR-146b-5p/TRAF6 axis is essential
for Ginkgo biloba L. extract GBE to attenuate
LPS-induced neuroinflammation. Front. Pharmacol. 2022, 13,
978587. https://doi.org/10.3389/fphar.2022.978587 |
|
31. |
Zhou, H.; Yang, R.K.; Li, Q.; Li, Z.; Wang, Y.C.; Li, S.Y.;
Miao, Y.; Sun, X.H.; Wang, Z. MicroRNA-146a-5p protects retinal ganglion
cells through reducing neuroinflammation in experimental glaucoma. Glia.
2024, 72(11), 2115-2141. https://doi.org/10.1002/glia.24600 |
|
32. |
Tamkovich, S.; Grigor’eva, A.; Eremina, A.; Tupikin, A.;
Kabilov, M.; Chernykh, V.; Vlassov, V.; Ryabchikova, E. What information can
be obtained from the tears of a patient with primary open angle glaucoma?
Clin. Chim. Acta. 2019, 495, 529–537. https://doi.org/10.1016/j.cca.2019.05.028 |
|
33. |
Han, P.; Sunada-Nara, K.; Kawashima, N.; Fujii, M.; Wang, S.;
Kieu, T.Q.; Yu, Z.; Okiji, T. MicroRNA-146b-5p Suppresses Pro-inflammatory
mediator synthesis via targeting TRAF6, IRAK1, and RELA in lipopolysaccharide-stimulated
human dental pulp cells. Int. J. Mol. Sci. 2023, 24(8), 7433. https://doi.org/
10.3390/ijms24087433 |
|
34. |
Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: an
overview. J. Nutr. Sci. 2016, 5, e47. https://doi.org/10.1017/jns.2016.41.
Erratum in: J. Nutr. Sci. 2025, 14, e11. https://doi.org/10.1017/jns.2024.73 |
|
35. |
Dini, S.; Zakeri, M.; Ebrahimpour, S.; Dehghanian, F.;
Esmaeili, A. Quercetin‑conjugated superparamagnetic iron oxide nanoparticles
modulate glucose metabolism-related genes and miR-29 family in the
hippocampus of diabetic rats. Sci. Rep. 2021, 11, 8618. https://doi.org/10.1038/s41598-021-87687-w |
|
36. |
Dalgaard, L.T.; Sørensen, A.E.; Hardikar, A.A.; Joglekar, M.V.
The microRNA-29 family: Role in metabolism and metabolic disease. Am. J.
Physiol. Cell. Physiol. 2022, 323(2), C367-C377. https://doi.org/10.1152/ajpcell.00051.2022 |
|
37. |
Lopez, N.N.; Rangan, R.; Clark, A.F.; Tovar-Vidales, T. Mirna
Expression in glaucomatous and TGFβ2 treated lamina cribrosa cells. Int. J.
Mol. Sci. 2021, 22(12), 6178. https://doi.org/10.3390/ijms22126178 |
|
38. |
Liu, H.; Xiu, Y.; Zhang, Q.; Xu, Y.; Wan, Q.; Tao, L. Silencing
microRNA‑29b‑3p expression protects human trabecular meshwork cells against
oxidative injury via upregulation of RNF138 to activate the ERK pathway. Int.
J. Mol. Med. 2021, 47, 101. https://doi.org/10.3892/ijmm.2021.4934 |
|
39. |
Zhu, Y.; Huang, Y.; Liu, M.; Yan, Q.; Zhao,
W.; Yang, P.; Gao, Q.; Wei, J.; Zhao, W.; Ma, L. Epigallocatechin gallate
inhibits cell growth and regulates miRNA expression in cervical carcinoma
cell lines infected with different high-risk human papillomavirus subtypes.
Exp. Ther. Med. 2019, 17(3), 1742-1748. https://doi.org/10.3892/etm.2018.7131 |
|
40. |
Zhao, Y.; Chen, X.; Jiang, J.;
Wan, X.; Wang, Y.; Xu, P. Epigallocatechin
gallate reverses gastric cancer by regulating the long noncoding RNA
LINC00511/miR-29b/KDM2A axis. Biochim. Biophys. Acta Mol. Basis. Dis. 2020,
1866(10), 165856. https://doi.org/10.1016/j.bbadis.2020.165856 |
|
41. |
Li, Y.; Dou, Q.P.; Li, B.; Wang, Y.; Wang, X.; Guan, H.; Lia,
D.; Li, F. MicroRNAs as novel molecular targets of green tea polyphenol
epigallocatechin-3-gallate (EGCG): relevance and importance to nutrition
sciences and cancer prevention. Food Sci. Hum. Wellness. 2026, 15.
https://doi.org/10.26599/FSHW.2024.9250317 |
|
42. |
Rajabi, S.; Najafipour, H.; Sheikholeslami, M.;
Jafarinejad-Farsangi, S.; Beik, A.; Askaripour, M.; Karam, Z.M. Perillyl
alcohol and quercetin modulate the expression of non-coding RNAs MIAT, H19,
miR-29a, and miR-33a in pulmonary artery hypertension in
rats. Non-coding RNA Res. 2022, 7(1), 27–33. https://doi.org/10.1016/j.ncrna.2022.01.005 |
|
43. |
Zhou, L.; Wang, L.; Lu, L.; Jiang, P.; Sun, H.; Wang, H.
Inhibition of miR-29 by TGF-beta-Smad3 signaling through dual mechanisms
promotes transdifferentiation of mouse myoblasts into
myofibroblasts. PloS One. 2012, 7(3), e33766. https://doi.org/
10.1371/journal.pone.0033766 |
|
44. |
Dostal, Z.; Modriansky, M. The effect of quercetin on microRNA
expression: A critical review. Biomed. Pap. Med. Fac. Univ. Palacky
Olomouc Czech Repub. 2019, 163(2), 95–106. https://doi.org/10.5507/bp.2019.030 |
|
45. |
Noratto, G.D.; Kim, Y.; Talcott, S.T.; Mertens-Talcott, S.U.
Flavonol-rich fractions of yaupon holly leaves (Ilex vomitoria,
Aquifoliaceae) induce microRNA-146a and have anti-inflammatory and
chemopreventive effects in intestinal myofibroblast CCD-18Co
cells. Fitoterapia. 2011, 82(4), 557–569. https://doi.org/
10.1016/j.fitote.2011.01.013 |
|
46. |
Dos Santos, B.L.; Dos Santos, C.C.; Soares, J.R.P.; da Silva,
K.C.; de Oliveira, J.V.R.; Pereira, G.S.; de Araújo, F.M.; Costa, M.F.D.;
David, J.M.; da Silva, V.D.A.; Butt, A.M.; Costa, S.L. The flavonoid
agathisflavone directs brain microglia/macrophages to a neuroprotective
anti-inflammatory and antioxidant state via regulation of NLRP3 inflammasome. Pharmaceutics. 2023,
15(5), 1410. https://doi.org/ 10.3390/pharmaceutics15051410 |
|
47. |
Dos Santos, B.L.; Dos Santos, C.C.; da Silva, K.C.; Nonaka,
C.K.V.; Souza, B.S.F.; David, J.M.; de Oliveira, J.V.R.; Costa, M.F.D.; Butt,
A.M.; da Silva, V.D.A.; Costa, S.L. The phytochemical agathisflavone
modulates miR146a and miR155 in activated microglia involving STAT3 signaling. Int.
J. Mol. Sci. 2024, 25(5), 2547. https://doi.org/10.3390/ijms25052547 |
|
48. |
Zhang, S.Q.; Obregon, D.; Ehrhart, J.; Deng, J.; Tian, J.; Hou,
H.; Giunta, B.; Sawmiller, D.; Tan, J. Baicalein reduces β-amyloid and
promotes nonamyloidogenic amyloid precursor protein processing in an
Alzheimer's disease transgenic mouse model. J. Neurosci. Res. 2013, 91(9),
1239-1246. https://doi.org/10.1002/jnr.23244 |
|
49. |
Li, H.L.; Shan, S.W.; Stamer, W.D.; Li, K.K.; Chan, H.H.; Civan,
M.M.; To, C.H.; Lam, T.C.; Do, C.W. Mechanistic effects of baicalein on
aqueous humor drainage and intraocular pressure. Int. J. Mol. Sci.
2022, 23(13), 7372. https://doi.org/ 10.3390/ijms23137372 |
|
50. |
Gao, Y.; Lu, J.; Zhang, Y.; Chen, Y.; Gu, Z.; Jiang, X.
Baicalein attenuates bleomycin-induced pulmonary fibrosis in rats through
inhibition of miR-21. Pulm. Pharmacol. Ther. 2013, 26(6), 649–654.
https://doi.org/ 10.1016/j.pupt.2013.03.006 |
|
51. |
Cui, X.; Sun, X.; Lu, F.; Jiang, X. Baicalein represses
TGF-β1-induced fibroblast differentiation through the inhibition of miR-21.
Toxicol. Appl. Pharmacol. 2018, 358, 35–42.
https://doi.org/10.1016/j.taap.2018.09.007 |
|
52. |
Su, W.; Li, Z.; Jia, Y.; Zhu, Y.; Cai, W.; Wan, P.; Zhang, Y.;
Zheng, S.G.; Zhuo, Y. microRNA-21a-5p/PDCD4 axis regulates mesenchymal stem
cell-induced neuroprotection in acute glaucoma. J. Mol. Cell. Biol. 2017,
9(4), 289-301. https://doi.org/10.1093/jmcb/mjx022. Erratum in: J. Mol. Cell.
Biol. 2023, 15(4), mjad028. https://doi.org/10.1093/jmcb/mjad028 |
|
53. |
Tan, C.; Jia, F.; Zhang, P.; Sun, X.; Qiao, Y.; Chen, X.; Wang,
Y.; Chen, J.; Lei, Y. A miRNA stabilizing polydopamine nano-platform for
intraocular delivery of miR-21-5p in glaucoma therapy. J. Mater. Chem. B.
2021, 9(15), 3335-3345. https://doi.org/10.1039/d0tb02881a. Erratum in: J. Mater.
Chem. B. 2021, 9(16), 3595. https://doi.org/10.1039/d1tb90052h |
|
54. |
Dong, N.; Xu, B.; Shi, H.; Tang, X. Baicalein inhibits
amadori-glycated albumin-induced MCP-1 expression in retinal ganglion cells
via a microRNA-124-dependent mechanism. Invest. Ophthalmol. Vis.
Sci. 2015, 56(10), 5844–5853. https://doi.org/10.1167/iovs.15-17444 |
|
55. |
Xiao, J.R.; Do, C.W.; To, C.H. Potential therapeutic effects of
baicalein, baicalin, and wogonin in ocular disorders. J. Ocul. Pharmacol.
Ther. 2014, 30(8), 605–614. https://doi.org/10.1089/jop.2014.0074 |
|
56. |
Zhao, N.; Shi, J.; Xu, H.; Luo, Q.; Li, Q.; Liu, M. Baicalin
suppresses glaucoma pathogenesis by regulating the PI3K/AKT signaling in
vitro and in vivo. Bioengineered. 2021, 12(2), 10187-10198.
https://doi.org/10.1080/ 21655979.2021.2001217 |
|
57. |
Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and
prooxidant properties of flavonoids. Fitoterapia. 2011, 82(4), 513–523.
https://doi.org/ 10.1016/j.fitote.2011.01.018 |
|
58. |
Zhang, F.; Li, T.; Wan, J.; Wang, L.; Guo, W.; Hu, Y.; Wang, H.;
Bian, W. Protective effect of resveratrol on retinal damage in glaucoma: a
systematic review and meta-analysis of preclinical studies. Front.
Pharmacol. 2025,15, 1521188. https://doi.org/
10.3389/fphar.2024.1521188 |
|
59. |
Yang, B.; Ma, S.; Wang, Y.B.; Xu, B.; Zhao, H.; He, Y.Y.; Li,
C.W.; Zhang, J.; Cao, Y.K.; Feng, Q.Z. Resveratrol exerts protective effects
on anoxia/reoxygenation injury in cardiomyocytes via miR-34a/Sirt1 signaling
pathway. Eur. Rev. Med. Pharmacol. Sci. 2016, 20(12), 2734–2741 |
|
60. |
Boshra, S.A. Resveratrol modulates miR-34a in cardiotoxicity
induced by isoproterenol. J. Med. Food. 2020, 23(6),
593–599. https://doi.org/ 10.1089/jmf.2019.0209 |
|
61. |
Keshavarzmotamed, A.; Mousavi, V.; Masihipour, N.; Rahmati, A.;
Mousavi Dehmordi, R.; Ghezelbash, B.; Alimohammadi, M.; Mafi, A. Regulating
miRNAs expression by resveratrol: Novel insights based on molecular mechanism
and strategies for cancer therapy. Curr. Mol. Pharmacol. 2023, 17,
e18761429249717. https://doi.org/10.2174/ 0118761429249717230920113227 |
|
62. |
Golmohammadi, M.; Meibodi, S.A.A.; Al-Hawary, S.I.S.; Gupta, J.;
Sapaev, I.B.; Najm, M.A.A.; Alwave, M.; Nazifi, M.; Rahmani, M.; Zamanian,
M.Y.; Moriasi, G. Neuroprotective effects of resveratrol on retinal ganglion
cells in glaucoma in rodents: A narrative review. Animal Model Exp. Med.
2024, 7(3), 195-207. https://doi.org/ 10.1002/ame2.12438 |
|
63. |
Mansouri, F.; Feliziani, G.; Bordoni, L.; Gabbianelli, R. Impact
of resveratrol supplementation on human sirtuin 1: A grading of recommendations
assessment, development and evaluation-assessed systematic review and dose-response
meta-analysis of randomized controlled trials. J. Acad. Nutr. Diet. 2025,
S2212-2672(25)00114-5. https://doi.org/10.1016/j.jand.2025.03.011 |
|
64. |
Esmaealzadeh, N.; Miri, MS.; Mavaddat, H.; Peyrovinasab, A.;
Ghasemi Zargar, S.; Sirous Kabiri, S.; Razavi, S.M.; Abdolghaffari, A.H. The
regulating effect of curcumin on NF-κB pathway in neurodegenerative diseases:
a review of the underlying mechanisms. Inflammopharmacology. 2024, 32(4),
2125-2151. https://doi.org/10.1007/s10787-024-01492-1 |
|
65. |
Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.;
Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye
diseases. Cent. Eur. J. Immunol. 2019, 44(2), 181-189. https://doi.org/10.5114/ceji.2019.87070 |
|
66. |
Ribeiro, A.; Oliveira, D.; Cabral-Marques, H. Curcumin in ophthalmology:
mechanisms, challenges, and emerging opportunities. Molecules. 2025, 30, 457.
https://doi.org/10.3390/molecules30030457 |
|
67. |
Cheng, Y.H.; Ko, Y.C.; Chang, Y.F.; Huang, S.H.; Ling, L.C.J.
Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded
nanoparticles and latanoprost as a dual-drug delivery system for glaucoma
treatment. Exp. Eye Res. 2019, 179,179–187. https://doi.org/10.1016/j.exer.2018.11.017 |
|
68. |
Chandrasekaran, P.R.; Madanagopalan, V.G. Role of curcumin in
retinal diseases-A review. Graefes Arch. Clin. Exp. Ophthalmol. 2022,
260(5), 1457–1473. https://doi.org/10.1007/s00417-021-05542-0 |
|
69. |
Yue, Y.K.; Mo, B.; Zhao, J.; Yu, Y.J.; Liu, L.; Yue, C.L.; Liu,
W. Neuroprotective effect of curcumin against oxidative damage in BV-2
microglia and high intraocular pressure animal model. J. Ocul. Pharmacol.
Ther. 2014, 30(8), 657-664. https://doi.org/ 10.1089/jop.2014.0022. |
|
70. |
Wang, L.; Li, C.; Guo, H.; Kern, T.S.; Huang, K.; Zheng, L.
Curcumin inhibits neuronal and vascular degeneration in retina after ischemia
and reperfusion injury. PLoS One. 2011, 6(8), e23194.
https://doi.org/10.1371/journal.pone.0023194 |
|
71. |
Zhou, C.; Zhao, L.; Wang, K.; Qi, Q.; Wang, M.; Yang, L.; Sun,
P.; Mu, H. MicroRNA-146a inhibits NF-κB activation and pro-inflammatory
cytokine production by regulating IRAK1 expression in THP-1 cells. Exp. Ther.
Med. 2019, 18(4), 3078-3084. https://doi.org/ 10.3892/etm.2019.7881 |
|
72. |
Wu, H.; Liu, Q.; Cai, T.; Chen, Y.D.; Wang, Z.F. Induction of
microRNA-146a is involved in curcumin-mediated enhancement of temozolomide
cytotoxicity against human glioblastoma. Mol. Med. Rep. 2015,
12(4), 5461–5466. https://doi.org/ 10.3892/mmr.2015.4087 |
|
73. |
Wang, J.; Qiu, Y.; Shi, N.W.; Zhao, J.N.; Wang, Y.C.; Jiang, H.;
Qian, H.B. microRNA-21 mediates the TGF-β1-induced migration of keratinocytes
via targeting PTEN. Eur. Rev. Med. Pharmacol. Sci. 2016, 20(18), 3748–3759. https://www.europeanreview.org/article/11443 |
|
74. |
Tang, C.; Lin, W.; Shao, X.; Guo, X. Effects of curcumin on miR-21/Tregs
and IL-6 in PBMCs from patients with myocardial infarction. J.
Cardiovasc. Pharmacol. Ther. 2025, 30, 10742484251351124.
https://doi.org/10.1177/10742484251351124 |
|
75. |
Tan, C.; Song, M.; Stamer, W.D.; Qiao, Y.; Chen, X.; Sun, X.;
Lei, Y.; Chen, J. miR-21-5p: A viable therapeutic strategy for regulating
intraocular pressure. Exp. Eye Res. 2020, 200, 108197. https://doi.org/10.1016/j.exer.2020.108197 |
|
76. |
Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J.
Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol.
Ther. 2012, 133(1), 79-97. https://doi.org/10.1016/j.pharmthera.2011.09.002 |
|
77. |
Tomida, I.; Pertwee, R.G.; Azuara-Blanco, A. Cannabinoids and
glaucoma. Br. J. Ophthalmol. 2004, 88(5), 708-13. https://doi.org/10.1136/bjo.2003.032250 |
|
78. |
Juknat, A.; Gao, F.; Coppola, G.; Vogel, Z.; Kozela, E. miRNA
expression profiles and molecular networks in resting and LPS-activated BV-2
microglia-effect of cannabinoids. PLoS One. 2019, 14(2), e0212039.
https://doi.org/ 10.1371/journal.pone.0212039 |
|
79. |
Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P.S.
Combination of cannabinoids, Δ9- tetrahydrocannabinol and cannabidiol,
ameliorates experimental multiple sclerosis by suppressing neuroinflammation
through regulation of miRNA-mediated signaling pathways. Front. Immunol.
2019, 10, 1921. https://doi.org/10.3389/fimmu.2019.01921 |
|
80. |
Joshi, N.; Mariam, H.; Kamath, A. Cannabinoids for the treatment
of glaucoma: a review. Med. Cannabis Cannabinoids. 2024, 7(1), 183–192. https://doi.org/10.1159/000541461 |
|
81. |
Cheng, Z.; Kang, C.; Che, S.; Su, J.; Sun, Q.; Ge, T.; Guo, Y.;
Lv, J.; Sun, Z.; Yang, W.; Li, B.; Li, X.; Cui, R. Berberine: A promising
treatment for neurodegenerative diseases. Front. Pharmacol. 2022, 13, 845591.
https://doi.org/ 10.3389/fphar.2022.845591 |
|
82. |
D'Angelo, A.; Vitiello, L.; Lixi, F.; Abbinante,
G.; Coppola, A.; Gagliardi, V.; Pellegrino, A.; Giannaccare, G. Optic nerve
neuroprotection in glaucoma: A narrative review. J. Clin. Med. 2024, 13(8),
2214. https://doi.org/
10.3390/jcm13082214 |
|
83. |
Chu-Tan, J.A.; Rutar, M.; Saxena, K.; Aggio-Bruce, R.; Essex,
R.W.; Valter, K.; Jiao, H.; Fernando, N.; Wooff, Y.; Madigan, M.C.; Provis,
J.; Natoli, R. MicroRNA-124 Dysregulation is associated with retinal
inflammation and photoreceptor death in the degenerating retina. Invest.
Ophthalmol. Vis. Sci. 2018, 59(10), 4094–4105. https://doi.org/10.1167/iovs.18-24623 |
|
84. |
Chen, Y.; Lin, J.; Schlotterer, A.; Kurowski, L.; Hoffmann, S.;
Hammad, S.; Dooley, S.; Buchholz, M.; Hu, J.; Fleming, I.; Hammes, H.P.
MicroRNA-124 alleviates retinal vasoregression via regulating microglial
polarization. Int. J. Mol. Sci. 2021, 22(20), 11068. doi:
10.3390/ijms222011068 |
|
85. |
Zhai, J.; Li, Z.; Zhang, H.; Ma, L.; Ma, Z.; Zhang, Y.; Zou, J.;
Li, M.; Ma, L.; Wang, X.; Li, X. Berberine protects against diabetic
retinopathy by inhibiting cell apoptosis via deactivation of the NF‑κB
signaling pathway. Mol. Med. Rep. 2020, 22(5), 4227-4235. https://doi.org/
10.3892/mmr.2020.11505 |
|
86. |
Zhu, C.; Li, J.; Hua, Y.; Wang, J.; Wang,
K.; Sun, J. Berberine inhibits the expression of SCT through miR-214-3p stimulation
in breast cancer cells. Evid. Based Complement. Alternat. Med. 2020, 2020,
2817147. https://doi.org/10.1155/2020/2817147 |
|
87. |
Luo, X.; Gu, J.; Zhu, R.; Feng, M.; Zhu, X.; Li,
Y.; Fei, J. Integrative analysis of differential miRNA and functional study
of miR-21 by seed-targeting inhibition in multiple myeloma cells in response
to berberine. BMC Syst. Biol. 2014, 8, 82. https://doi.org/10.1186/1752-0509-8-82 |
|
88. |
Lin, C.Y.; Hsieh, P.L.; Liao, Y.W.; Peng, C.Y.;
Lu, M.Y.; Yang, C.H.; Yu, C.C.; Liu, C.M. (2017). Berberine-targeted miR-21
chemosensitizes oral carcinomas stem cells. Oncotarget.
2017, 8(46), 80900–80908. https://doi.org/10.18632/ oncotarget.20723 |
|
89. |
Witwer, K.W.; McAlexander, M.A.; Queen, S.E.; Adams, R.J.
Real-time quantitative PCR and droplet digital PCR for plant miRNAs in
mammalian blood provide little evidence for general uptake of dietary miRNAs:
limited evidence for general uptake of dietary plant xenomiRs. RNA Biol.
2013, 10(7), 1080-6. https://doi.org/10.4161/rna.25246 |
|
90. |
Philip, A.; Ferro, V.A.; Tate, R.J. Determination of the
potential bioavailability of plant microRNAs using a simulated human
digestion process. Mol. Nutr. Food Res. 2015, 59(10), 1962–1972.
https://doi.org/10.1002/mnfr.201500137 |
|
91. |
Zhao, Q., Liu,
Y., Zhang, N., Hu, M., Zhang, H., Joshi, T., Xu, D. Evidence for
plant-derived xenomiRs based on a large-scale analysis of public small RNA
sequencing data from human samples. PLoS One. 2018, 13(6), e0187519.
https://doi.org/ 10.1371/journal.pone.0187519. Erratum in: PLoS One. 2019,
14(10), e0224537. https://doi.org/10.1371/journal.pone.0224537 |
|
92. |
Díez-Sainz, E.; Milagro, F.I.; Aranaz, P.; Riezu-Boj, J.I.;
Lorente-Cebrián, S. MicroRNAs from edible plants reach the human
gastrointestinal tract and may act as potential regulators of gene
expression. J. Physiol. Bioochem. 2024, 80(3), 655–670. https://doi.org/10.1007/s13105-024-01023-0 |
|
93. |
Mar-Aguilar, F.; Arreola-Triana, A.; Mata-Cardona, D.;
Gonzalez-Villasana, V.; Rodríguez-Padilla, C.; Reséndez-Pérez, D. Evidence of
transfer of miRNAs from the diet to the blood still inconclusive. PeerJ.
2020, 8, e9567. https://doi.org/10.7717/peerj.9567 |
|
94. |
Yang, L.; Feng, H. Cross-kingdom regulation by plant-derived
miRNAs in mammalian systems. Animal Model Exp. Med. 2023, 6(6), 518-525. https://doi.org/
10.1002/ame2.12358 |
|
95. |
Liu, R.; Zhang, F.; He, X.; Huang, K. Plant derived exosome-like
nanoparticles and their therapeutic applications in glucolipid metabolism
diseases. J. Agric. Food Chem. 2025, 73(11), 6385-6399. https://doi.org/10.1021/acs.jafc.4c12480 |
|
96. |
Zhao, Q.; Mao,
Q.; Zhao, Z.; Dou, T.; Wang, Z.; Cui, X.; Liu, Y.; Fan, X. Prediction of
plant-derived xenomiRs from plant miRNA sequences using random forest and
one-dimensional convolutional neural network models. BMC Genomics. 2018,
19(1), 839. https://doi.org/10.1186/s12864-018-5227-3 |
|
97. |
Dos Santos,
J.A.C.; Veras, A.S.C.; Batista, V.R.G.; Tavares, M.E.A.; Correia, R.R.;
Suggett, C.B.; Teixeira, G.R. Physical exercise and the functions of
microRNAs. Life Sci. 2022, 304, 120723. https://doi.org/10.1016/j.lfs.2022.120723 |
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
Glaucoma is a progressive
optic neuropathy and a leading cause of irreversible blindness, characterized
by retinal ganglion cell loss and optic nerve degeneration. While elevated
intraocular pressure (IOP) is the main modifiable risk factor, disease
progression can occur independently of IOP, implicating oxidative stress,
inflammation, mitochondrial dysfunction, and extracellular matrix remodeling in
its pathogenesis. MicroRNAs (miRNAs), small non-coding RNAs that regulate gene
expression, have emerged as promising modulators of these pathways and
potential therapeutic targets, although clinical application remains limited by
delivery challenges and variable findings. Emerging evidence suggests that
certain natural compounds and plant-derived bioactive agents can influence
miRNA activity, with potential neuroprotective effects. Although direct
evidence in glaucoma is still limited, studies in related neurodegenerative and
inflammatory conditions indicate a promising therapeutic avenue. This review
discusses the potential of miRNA-targeted phytotherapy as a novel strategy to
modulate key pathogenic pathways and enhance neuroprotection in glaucoma.
Future research should focus on standardized methodologies and robust clinical
validation to translate these findings into therapeutic applications.
Abstract Keywords
MicroRNA, glaucoma, flavonoids, resveratrol, curcumin, cannabinoids, berberine, Ginkgo biloba.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).

